Drug Access Protocol (DAP)
A Dutch National Protocol to Facilitate Patient Access to Novel Anti-cancer Drugs Awaiting Regulatory Approval or Reimbursement
Enrollment
Recruiting
No. of patients
Not applicable
Population
Adult and pediatric patients that have solid cancer and acceptable performance status and organ function
Design
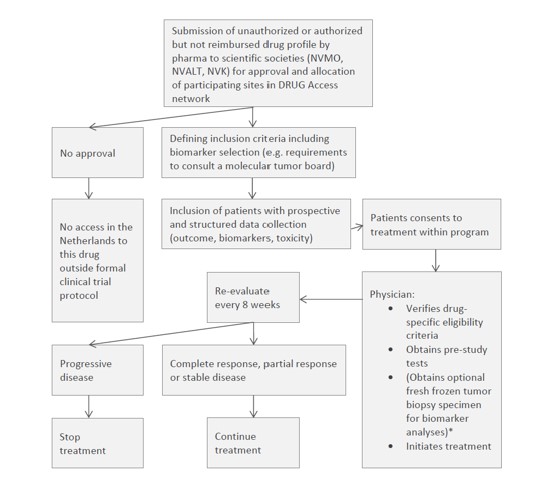
Prospective, open-label, non-randomized data collection trial. Patients will be enrolled in multiple parallel cohorts, each defined by the novel unauthorised drug and its requested label after authorisation or the authorised, not (yet) reimbursed drug with its registered indication
Key outcome parameters
Primary endpoints:
- To enable and assist oncologists to prescribe unauthorized anticancer drugs used for treatment of patients with solid tumors, awaiting FDA/EMA approval or authorized anticancer drugs, awaiting approval of reimbursement in the Netherlands
- To collect real-world evidence on the anti-tumor activity and toxicity of these unauthorised anti-cancer drugs awaiting FDA/EMA approval and of authorized anticancer drugs that are awaiting reimbursement in the Netherlands used for treatment of adult and pediatric patients with solid tumors
Secondary endpoint:
- To perform biomarker analyses, including (but not limited to) next generation sequencing on fresh tumor biopsy specimen
Intervention
Key inclusion criteria
Inclusion criteria:
- Solid cancer and acceptable performance status and organ function. For authorised indications or for drugs with a positive CHMP opinion, the eligibility and age restriction will be based on the (proposed) EMA label. If the drug specifically targets a molecular profile, a genomic or protein expression test must have been performed on the tumor and results must identify this potentially actionable molecular profile.
- Objectively evaluable or measurable disease (by physical or radiographic examination), according to RECIST v1.1 for patients with solid tumors. If a patient fulfils all drug-specific criteria but does not meet the criterion of measurable disease according to one of the above-mentioned evaluation protocols, patients can still be included in the study. In that case, method of treatment evaluation will be decided by the treating physician after consultation of central study team.
- If the drug specifically targets a molecular profile, results must be available from a tumor genomic or protein expression test. Eligible tests may include: FISH, PCR, CGH, NGS or IHC. The test results (full pathology or molecular diagnostics report) must be uploaded in the eCRF.
- Patients must have a tumor profile for which treatment with an unauthorized drug awaiting FDA/EMA approval or with an authorized anticancer drug that is awaiting reimbursement in the Netherlands has potential clinical benefit based on clinical data.
- In case patient agrees with obtaining a pre-treatment biopsy: a new (obtained ≤2 months before inclusion, and without any type of anti-cancer therapy within those ≤2 months) fresh frozen tumor biopsy specimen for extensive biomarker testing is taken before the start of treatment with a targeted agent included in the protocol. To patients who do not agree with tumor biopsy, this criterion does not apply. For children this will only be performed when needed as standard of care.
- Ability to understand and the willingness to sign a written informed consent document.
Key exclusion criteria
Exclusion criteria:
- Ongoing toxicity from prior anti-cancer therapies > grade 2, other than alopecia.
- Patient is receiving any other anti-cancer therapy (cytotoxic, biologic, radiation, or hormonal other than for replacement) that is not part of the label (i.e. intented combination therapy is allowed). Specific combinations specified in the drug-specific label however will take precedence. Required wash out period prior to starting treatment within the protocol is at least two weeks. An exception is made for:
- Patients suffering from CRPC are allowed to continue androgen deprivation therapy.
- Medications that are prescribed for supportive care but may potentially have an anti-cancer effect (e.g., megestrol acetate, bisphosphonates). These medications must have been started ≥ 1 week prior to enrollment on this study.
- Patient is pregnant or nursing.
Contact opnemen over een studie
Neem contact op voor meer informatie over de studies van de afdeling thoracale oncologie van Amsterdam UMC.